In the fast-paced world of pharmaceutical innovation, an often overlooked, yet pivotal challenge is data management. For our client, a global pharmaceutical company, this was not merely a theoretical obstacle, but a substantial barrier. Hindered by fragmented data repositories, limited access to crucial data sources, and inconsistent data structures across clinical trials, the journey towards breakthroughs was daunting. These obstacles posed significant challenges to extracting valuable insights and expediting drug development.
The Challenge in Pharmaceutical Innovation
Introducing a Data Insights Program
Despite data management being a challenge for many pharmaceuticals, our client rose to the occasion, launching a data insights program – a game-changing initiative in R&D data insights. Conceived as a one-stop shop for clinical data, the program is designed to streamline data storage, curation, analysis, and visualisation, revolutionising how data informs decision-making.
The Approach and Implementation
The data insights program, anchored in the Data Science division, uses robust data pipelines and models across diverse data modalities, aiming to create a solution that permeates the entire value chain. This pioneering project is set to turbocharge drug development processes, marking a significant stride in our client’s innovation journey.
Collaboration with ADC
To address these challenges, our client partnered with our team at ADC, drawing on their expertise in data engineering and cloud architecture. Together, we embarked on a journey to overhaul data management practices. From the project’s inception, our team provided technical guidance, ensuring a solid foundation for the data insights program by harmonising clinical trial data in Study Data Tabulation Model (SDTM) format and enabling Analysis Data Model (ADaM) from Clinical Data Interchange Standards Consortium (CDISC).
The Culmination: A Data Insights Platform
The collaborative effort culminated in the development of the data insights platform—a seamless infrastructure where all data from completed clinical trials are collected and prepared for insights-generation. By integrating various data types, including real-world data, omics, and imaging, the platform empowers stakeholders with comprehensive insights.
Built on a scalable cloud architecture, the platform offers streamlined access to harmonised data products, facilitating informed decision-making and accelerating drug development timelines. Moreover, the platform is designed to be automated, self-updating, and sustainable, ensuring long-term effectiveness.
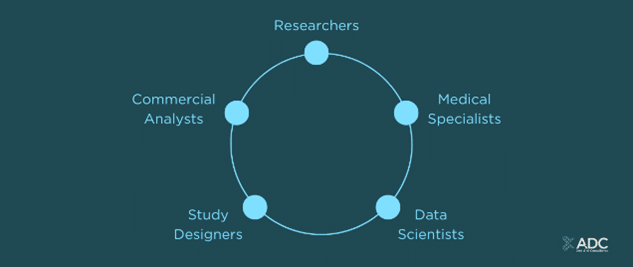
The platform democratises data across our client’s value chain, empowering researchers, medical specialists, data scientists, study designers, and commercial analysts.
Impact on our Client
The implementation of the data insights platform represents a notable advancement for the pharmaceutical company. Formerly fragmented data repositories and inconsistent structures across clinical trials have been replaced with a unified platform, fostering collaboration and efficiency. The platform ensures easy access to clinical trial data in a seamless yet controlled manner, making it effortless for our client to find available data from various sources including real-world data, omics, and imaging.
Additionally, the platform facilitates the connection of multiple data types and sources across the organisation, empowering informed decision-making and accelerating drug development processes. Ultimately, this platform reinforces our client’s commitment to enhancing patient care through innovation and collaboration.
Continue the Conversation
Are you interested in discovering how ADC can assist your life science organisation in optimising data management process? Our team has extensive experience in developing solutions that create real impact. To learn more, please contact Andreas Kjær (Life Sciences Lead).

What stage is your organisation in on its data-driven journey?
Discover your data maturity stage. Take our Data Maturity Assessment to find out and gain valuable insights into your organisation’s data practices.